Inflammatory Bowel Disease

Combines serologic, genetic, and inflammation markers to help differentiate IBD vs non-IBD and ulcerative colitis vs Crohn’s disease.
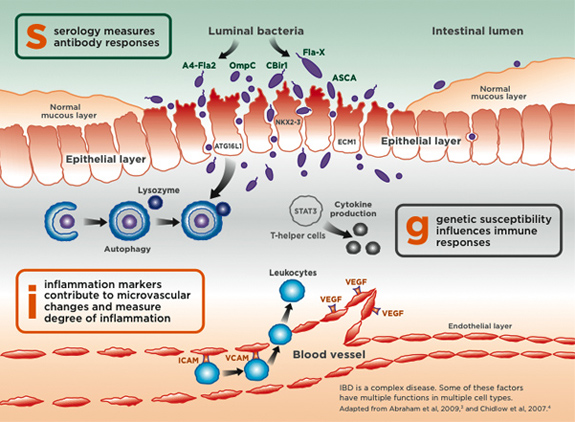
The PROMETHEUS® IBD sgi Diagnostic® is the 4th-generation IBD diagnostic test and the first and only test to combine serologic, genetic, and inflammation markers in the proprietary Smart Diagnostic Algorithm for added diagnostic clarity. This test aids healthcare providers in differentiating IBD vs non-IBD and CD vs UC in one comprehensive blood test. This assay includes 9 serological markers ASCA IgA, ASCA IgG and proprietary markers anti-Fla-X, anti-A4-Fla2, anti-CBir1, anti-OMPC, and DNAse-sensitive pANCA that helps identify patients with IBD and utilizes Smart Diagnostic Algorithm Technology to improve the predictive accuracy. Genetic susceptibility influences immune responses and this assay includes evaluation of ATG16L1, STAT3, NKX2-3, and ECM1. Inflammatory markers include VEGF, ICAM-1, VCAM-1, CRP, SAA. While most other labs only offer assay values, PROMETHEUS® IBD sgi Diagnostic® provides added clarity in diagnosing IBD, UC, and CD.
Sample insurance correspondence for PROMETHEUS® IBD sgi Diagnostic®
References
Plevy, Silverberg, Lockton et al. Combined archaeology, genetic, and inflammatory markers differentiate non-IBD, Crohn’s Disease, and ulcerative colitis patients. Inflammatory Bowel Diseases. 2013; 19(6):1139-48.
Lichtenstein, Targan, Dubinsky et al. Combination of genetic and quantitative serological immune markers are associated with complicated Crohn’s Disease behavior. Inflammatory Bowel Diseases. 2011; 17(12):2488-96.
Chidlow, Jr., Shukla, Grisham et al. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. American Journal of Physiology.Gastrointestinal and Liver Physiology. 2007;293:G5-G18.
Cummings, Cooney, Pathan et al. Confirmation of the role of ATG16L1 as a Crohn’s disease susceptibility gene. Inflammatory Bowel Diseases. 2007;13:941-946.
Henriksen, Jahnsen, Lygren et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518-1523.
Meggyesi, Kiss, Koszarska et al. NKX2-3 and IRGM variants are associated with disease susceptibility to IBD in Eastern European patients. World J.Gastroenterol. 2010;16:5233-5240.
Sandborn, Loftus, Colombel, et al. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn’s disease. Inflammatory Bowel Diseases. 2001; 7:192-201.
Schoepfer, Schaffer, Mueller et al. Phenotypic associations of Crohn’s disease with antibodies to flagellins A4-Fla2 and Fla-X, ASCA, p-ANCA, PAB, and NOD2 mutations in a Swiss Cohort. Inflammatory Bowel Diseases. 2009;15:1358-1367.
Targan, Landers, Yang et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020-2028.
Thompson, Lees. Genetics of ulcerative colitis. Inflammatory Bowel Diseases. 2011;17:831-848.
Lees, Satsangi. Genetics of inflammatory bowel disease: implications for disease pathogenesis and natural history. Expert Rev. Gastroenterol.Hepatol. 2009;3:513-534.
These tests have not been cleared or approved by the US FDA. The tests are used for clinical purposes and should not be regarded as investigational or for research. Prometheus Laboratories is certified under the Clinical Laboratory Improvement Amendments (CLIA) as qualified to perform high-complexity clinical laboratory testing and is accredited by the College of American Pathologists (CAP).
This material is provided for general information purposes only as an educational service for healthcare physicians and their patients. It is not intended as a substitute for medical advice and/or consultation with a physician.
