Inflammatory Bowel Disease

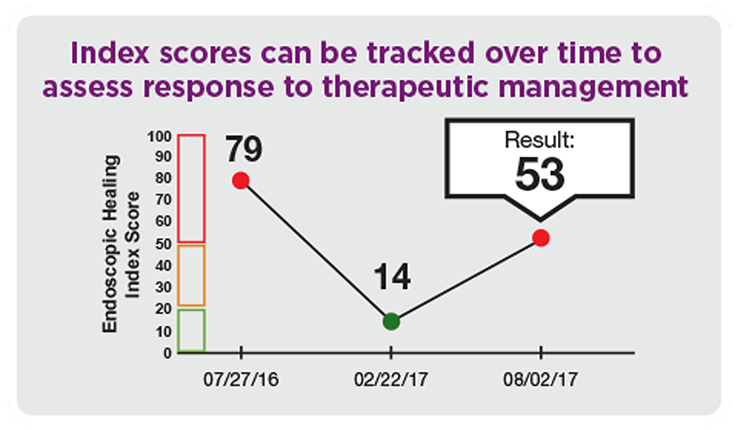
Measure & monitor mucosal healing changes over time
Improvement in mucosal damage provides an objective measure of therapeutic response in CD management.1 However, current assessment methods may not provide needed information, particularly when evaluating disease activity in difficult-to-reach locations to assess ideal disease.1-3
The Monitr® Crohn’s Disease Test is a laboratory-developed test that evaluates multiple markers of mucosal damage and repair processes, regardless of disease location.4 It applies a proprietary algorithm to 13 biomarkers to produce a quantitative Endoscopic Healing Index (EHI) Score—ranging from 0 to 100—to aid in distinguishing endoscopic remission from active disease in adult CD patients.
The next evolution
of the treat-to-target paradigm
In Crohn’s disease (CD) patient management, endoscopic healing is known to be an objective measure of therapeutic response.3 This is likely due to the fact that, in multiple clinical trials, endoscopic healing is a therapeutic goal that has been associated with improved long-term outcomes in CD patients3-6.
However, current assessment methods can make it challenging to:
- Noninvasively evaluate the endoscopic disease activity of your adult CD patients7,8
- Assess active disease in difficult-to-reach locations, including the ileum3,6,8
- Obtain objective results for individual patients7
- Regularly monitor patient response to therapy7
Measure and monitor the endoscopic disease activity of your adult CD patients with 1 simple serum test
The Monitr Crohn’s Disease Test is a laboratory-developed test that provides critical information to aid in:
- Establishing a baseline measurement of disease activity at initial presentation
- Tracking and assessing disease activity and response to therapeutic management
- Optimizing treatment management decisions
- Evaluating if treatment changes may be appropriate
- Counseling patients about therapeutic management goals
A powerful monitoring tool for CD patients.
Easy to order: Monitr Test Requisition Form. For assistance ordering,
contact Client Services toll-free in the US at 888-423-5227 (Monday through Friday from 6 AM to 4:30 PM PT).
References
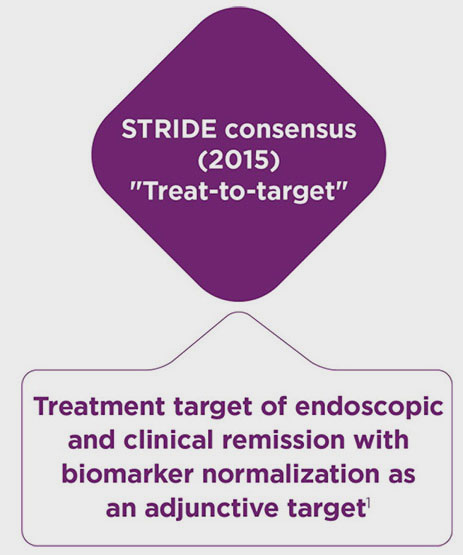
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324-1338.
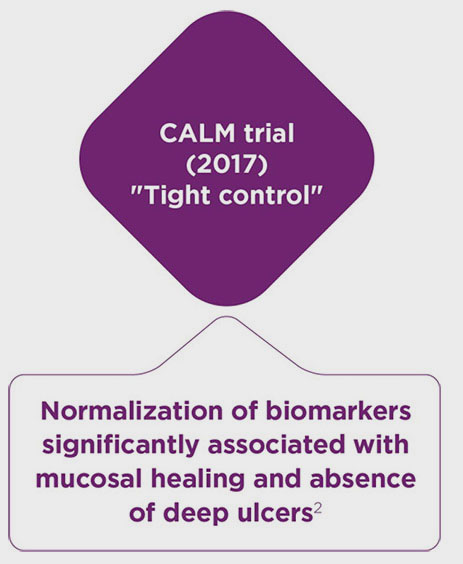
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779-2789
- De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2013;19(2):429-444.
- D’Haens G, Kelly O, Battat R, et al. Development and validation of a test to monitor endoscopic activity in patients with Crohn’s disease based on serum levels of proteins. Gastroenterology. 2020;158(3):515-526.e10.
- Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142(5):1102-1111.e2.
- Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43(3):317-333.
- Freeman HJ. Limitations in assessment of mucosal healing in inflammatory bowel disease. World J Gastroenterol. 2010;16(1):15-20.
- Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8(12):1582-1597.

